how does nitrobenzene reacts in acidic and basic medium
Are you a teacher? The electrolytic reduction of nitrobenzene in strongly acidic medium produces, When acetylen reacted with hydroxylic acid in presence of. Watch the recordings here on Youtube! Nitrobenezen electrolytic reduction in strongly acidic medium: Electrolytic reduction of nitrobenzene in weakly acidic medium gives aniline but in strongly acidic medium, it gives p – aminophenol obviously through the acid – cataLysed rearrangement of the initially formed phenyihydroxylamine. CBSE 2021 exam tips will definitely help you to score the highest marks in the board exam. to Euclids Geometry, Areas Identify compound X in the following sequence of reactions: Identify a molecule which does not exist. In the oxidation half reaction, one or more electrons are lost, while in the reduction half reaction, one or more electrons are gained. There are two electrophilic carbons in the epoxide, but the best target for the nucleophile in an SN2 reaction is the carbon that is least hindered. Redox reactions are those that involve both oxidation and reduction. On the other hand, if the reaction took place in an alkaline medium, hydroxyl ion (or `OH^-` ) ion will appear in either sides of the redox reaction equation. and Inverse Proportions, Areas The reading describes the relative reactivities of “biologically relevant acyl groups.” Both acid anhydrides and acid halides readily react with water and cannot exist for any length of time in living organisms. to Trigonometry, Complex Given the following, predict the product assuming only the epoxide is affected. The particular pH at which a given amino acid exists in solution as a zwitterion is called the isoelectric point (pI). Related to Circles, Introduction Benzene is treated with a mixture of concentrated nitric acid and concentrated sulphuric acid at a temperature not exceeding 50°C. General Reactions of Carbonyl Compounds.” Review if necessary. This depends on how much electron density the neighboring heteroatom on the acyl X group is able to donate: greater electron donation by the heteroatom implies lower partial positive charge on the carbonyl carbon, which in turn implies lower electrophilicity. If you are at an office or shared network, you can ask the network administrator to run a scan across the network looking for misconfigured or infected devices. Clemmensen reduction is complementary to Wolff-Kishner reduction, which also converts aldehydes and ketones to hydrocarbons, in that the former is carried out in strongly acidic conditions and the latter in strongly basic conditions. Like in other S N 2 reactions, nucleophilic reactions take place with backside orientation relative to the leaving group, resulting in inversion at the electrophilic carbon. You may need to download version 2.0 now from the Chrome Web Store. Know NCERT guidelines to safe & ethical use of online platforms by the students. Unlike in an SN1 reaction, the nucleophile reacts with the electrophilic carbon (step 3) before a complete carbocation intermediate has a chance to form. Educators go through a rigorous application process, and every answer they submit is reviewed by our in-house editorial team. Apne doubts clear karein ab Whatsapp (8 400 400 400) par The following scheme illustrates the relative reactivities of most carboxylic acid derivatives that you will encounter. Legal. When the incoming nucleophile in an acyl substitution is a water molecule, the reaction is also referred to as an acyl hydrolysis. ©2020 eNotes.com, Inc. All Rights Reserved. A thioester is more reactive than an ester, for example, because a thiolate (RS-) is a weaker base than an alkoxide (RO-). The magnesium ion also balances negative charge on the phosphate, making it an excellent leaving group. Predict the product of the following, similar to above but a different nucleophile is used and not in acidic conditions. These are most often present in two forms: the simple acyl monophosphate, and the acyl-adenosine monophosphate. The relative reactivity of the carboxylic acid derivatives is an important concept to understand before entering into a detailed examination of nucleophilic acyl substitutions. Zinc liberates hydrogen gas when reacted with dilute hydrochloric acid, whereas copper does not. What are 5 pure elements that can be found in your home? Predict the major product(s) of the ring opening reaction that occurs when the epoxide shown below is treated with: Hint: be sure to consider both regiochemistry and stereochemistry! The nonenzymatic ring-opening reactions of epoxides provides an oppourtunity to review the nucelophilic substitution mechansims. Nitrobenzene when reacted with tin and hydrochloric acid, i.e., in acidic medium, the product formed is: Nitrobenzene is an organic compound with the chemical formula C 6 H 5 NO 2.It is a water-insoluble pale yellow oil with an almond-like odor.It freezes to give greenish-yellow crystals. Nitrobenzene is strongest acid due to -M effect of nitro group and Phenol is also acidic in nature due to stability of phenolate ion. Know exam pattern, new marking scheme, sample paper & more. MP Board Exam 2021 - MPBSE Reduces Class 10, 12 Syllabus by 30 Percent. What this means is that the tetrahedral product formed from attack of the nucleophile on the carbonyl carbon is not the product: it is a reactive intermediate. If the following alkene were reacted with an oxyacid to form an epoxide, would the result be a enantiomerically pure? If the redox reaction takes place in an acidic medium, proton (or `H^+` ) will appear in the chemical equation for redox reaction. This accounts for the observed regiochemical outcome. Which product is formed when 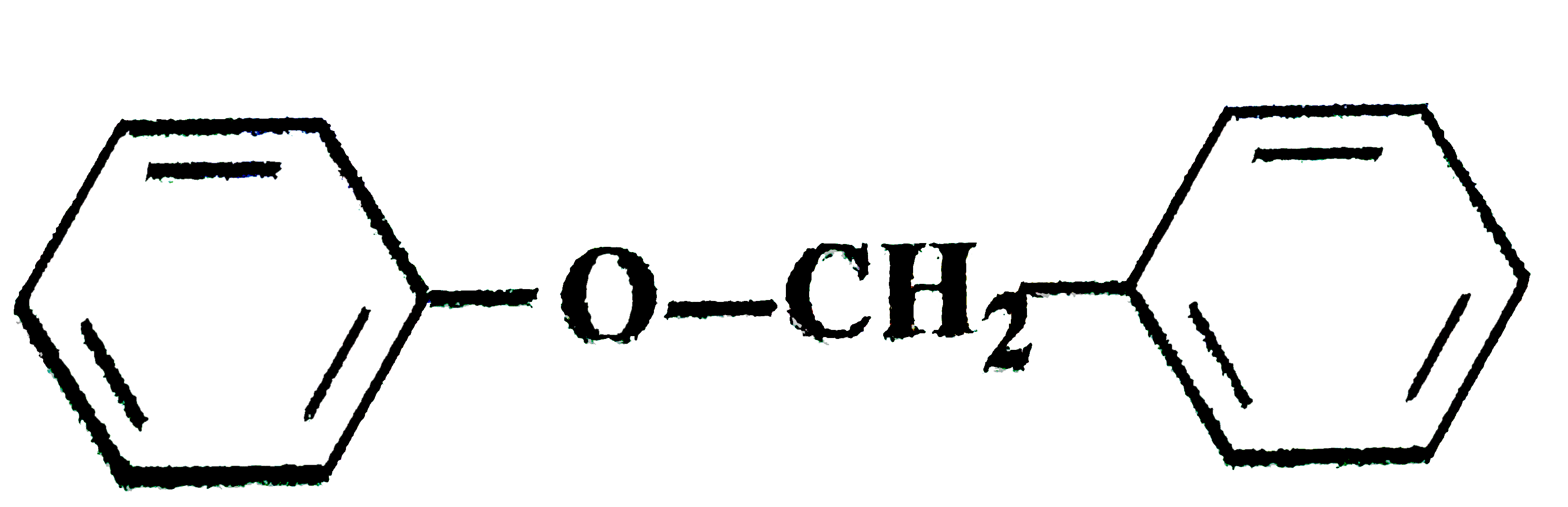 is reacted with HI ? When a nucleophilic substitution reaction involves a poor leaving group and a powerful nucleophile, it is very likely to proceed by an SN2 mechanism. These are both good examples of regioselective reactions. Both acid anhydrides and acid halides readily react with water and cannot exist for any length of time in living organisms. Both are highly reactive to acyl substitution reactions, and are often referred to as ‘activated acyl groups’ or ‘activated carboxylic acids’. The mechanism for the Sn\HCl reduction involves reductive electron transfer of the nitro group while the tin is being oxidized. ), Virtual Textbook of Organic Chemistry. Aniline 3. CBSE board exam 2021 preparation tips to ace the exam. These reactions can take place in either acidic or basic or neutral medium. explain the difference in reactivity towards nucleophiles of two or more given carboxylic acid derivatives. This leads to "two" epoxides. 2. Phenol. Like in other SN2 reactions, nucleophilic reactions take place with backside orientation relative to the leaving group, resulting in inversion at the electrophilic carbon. Carboxylic acid derivatives and acyl groups. Practice and master your preparation for a specific topic or chapter. The presence of these ions is used to balance the redox reaction equation. Which of the following set of molecules will have zero dipole moment ? The most reactive of the carboxylic acid derivatives frequently found in biomolecules are the acyl phosphates. As a general rule, the carbonyl carbon in an acyl group is less electrophilic than that in an aldehyde or ketone. As we will see in later sections of this chapter the hydrolysis of esters and amides are particularly important reaction types in biochemical pathways. There is a mirror plane, shown here. Delhi Govt. Identify product when (R) - and (S) -2 - butanol reacts with (R, R) tartaric acid in acidic medium. bhi. On electrolysis of dil.sulphuric acid using Platinum (Pt) electrode, the product obtained at anode will be: An element has a body centered cubic (bcc) structure with a cell edge of 288 pm. Common benzene reactions are Nitration of Benzene. Time it out for real assessment and get your results instantly. Ring-opening reactions can proceed by either SN2 or SN1 mechanisms, depending on the nature of the epoxide and on the reaction conditions. We will then discuss how thioesters play a key role in the acyl substitution reactions of lipid metabolism. 3. Electrolytic reduction of nitrobenzene in weakly acidic medium gives : Nitrobenzene on electrolytic reduction in strongly acidic medium gives, JEE Main 2021: January Session likely to be postponed to February. Chemistry . However, these two mirror images are actually identical due to the mirror plane of the cis geometry. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. This reaction is known as nitration of benzene. • Amides do undergo acyl substitution reactions in biochemical pathways, but these reactions are inherently slow and the enzymes catalyzing them have evolved efficient strategies to lower the activation energy barrier. Cloudflare Ray ID: 5f7c3ed9ceaf2c76 Notice, however, the regiochemical outcome is different from the base-catalyzed reaction. The amine that reacts will Hinsberg's reagent to give an alkali insoluble product is : Assertion: $(CH_3)_2NH$ is less basic than $(CH_3)_3N$ in aqueous solution.
is reacted with HI ? When a nucleophilic substitution reaction involves a poor leaving group and a powerful nucleophile, it is very likely to proceed by an SN2 mechanism. These are both good examples of regioselective reactions. Both acid anhydrides and acid halides readily react with water and cannot exist for any length of time in living organisms. Both are highly reactive to acyl substitution reactions, and are often referred to as ‘activated acyl groups’ or ‘activated carboxylic acids’. The mechanism for the Sn\HCl reduction involves reductive electron transfer of the nitro group while the tin is being oxidized. ), Virtual Textbook of Organic Chemistry. Aniline 3. CBSE board exam 2021 preparation tips to ace the exam. These reactions can take place in either acidic or basic or neutral medium. explain the difference in reactivity towards nucleophiles of two or more given carboxylic acid derivatives. This leads to "two" epoxides. 2. Phenol. Like in other SN2 reactions, nucleophilic reactions take place with backside orientation relative to the leaving group, resulting in inversion at the electrophilic carbon. Carboxylic acid derivatives and acyl groups. Practice and master your preparation for a specific topic or chapter. The presence of these ions is used to balance the redox reaction equation. Which of the following set of molecules will have zero dipole moment ? The most reactive of the carboxylic acid derivatives frequently found in biomolecules are the acyl phosphates. As a general rule, the carbonyl carbon in an acyl group is less electrophilic than that in an aldehyde or ketone. As we will see in later sections of this chapter the hydrolysis of esters and amides are particularly important reaction types in biochemical pathways. There is a mirror plane, shown here. Delhi Govt. Identify product when (R) - and (S) -2 - butanol reacts with (R, R) tartaric acid in acidic medium. bhi. On electrolysis of dil.sulphuric acid using Platinum (Pt) electrode, the product obtained at anode will be: An element has a body centered cubic (bcc) structure with a cell edge of 288 pm. Common benzene reactions are Nitration of Benzene. Time it out for real assessment and get your results instantly. Ring-opening reactions can proceed by either SN2 or SN1 mechanisms, depending on the nature of the epoxide and on the reaction conditions. We will then discuss how thioesters play a key role in the acyl substitution reactions of lipid metabolism. 3. Electrolytic reduction of nitrobenzene in weakly acidic medium gives : Nitrobenzene on electrolytic reduction in strongly acidic medium gives, JEE Main 2021: January Session likely to be postponed to February. Chemistry . However, these two mirror images are actually identical due to the mirror plane of the cis geometry. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. This reaction is known as nitration of benzene. • Amides do undergo acyl substitution reactions in biochemical pathways, but these reactions are inherently slow and the enzymes catalyzing them have evolved efficient strategies to lower the activation energy barrier. Cloudflare Ray ID: 5f7c3ed9ceaf2c76 Notice, however, the regiochemical outcome is different from the base-catalyzed reaction. The amine that reacts will Hinsberg's reagent to give an alkali insoluble product is : Assertion: $(CH_3)_2NH$ is less basic than $(CH_3)_3N$ in aqueous solution.
 is reacted with HI ? When a nucleophilic substitution reaction involves a poor leaving group and a powerful nucleophile, it is very likely to proceed by an SN2 mechanism. These are both good examples of regioselective reactions. Both acid anhydrides and acid halides readily react with water and cannot exist for any length of time in living organisms. Both are highly reactive to acyl substitution reactions, and are often referred to as ‘activated acyl groups’ or ‘activated carboxylic acids’. The mechanism for the Sn\HCl reduction involves reductive electron transfer of the nitro group while the tin is being oxidized. ), Virtual Textbook of Organic Chemistry. Aniline 3. CBSE board exam 2021 preparation tips to ace the exam. These reactions can take place in either acidic or basic or neutral medium. explain the difference in reactivity towards nucleophiles of two or more given carboxylic acid derivatives. This leads to "two" epoxides. 2. Phenol. Like in other SN2 reactions, nucleophilic reactions take place with backside orientation relative to the leaving group, resulting in inversion at the electrophilic carbon. Carboxylic acid derivatives and acyl groups. Practice and master your preparation for a specific topic or chapter. The presence of these ions is used to balance the redox reaction equation. Which of the following set of molecules will have zero dipole moment ? The most reactive of the carboxylic acid derivatives frequently found in biomolecules are the acyl phosphates. As a general rule, the carbonyl carbon in an acyl group is less electrophilic than that in an aldehyde or ketone. As we will see in later sections of this chapter the hydrolysis of esters and amides are particularly important reaction types in biochemical pathways. There is a mirror plane, shown here. Delhi Govt. Identify product when (R) - and (S) -2 - butanol reacts with (R, R) tartaric acid in acidic medium. bhi. On electrolysis of dil.sulphuric acid using Platinum (Pt) electrode, the product obtained at anode will be: An element has a body centered cubic (bcc) structure with a cell edge of 288 pm. Common benzene reactions are Nitration of Benzene. Time it out for real assessment and get your results instantly. Ring-opening reactions can proceed by either SN2 or SN1 mechanisms, depending on the nature of the epoxide and on the reaction conditions. We will then discuss how thioesters play a key role in the acyl substitution reactions of lipid metabolism. 3. Electrolytic reduction of nitrobenzene in weakly acidic medium gives : Nitrobenzene on electrolytic reduction in strongly acidic medium gives, JEE Main 2021: January Session likely to be postponed to February. Chemistry . However, these two mirror images are actually identical due to the mirror plane of the cis geometry. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. This reaction is known as nitration of benzene. • Amides do undergo acyl substitution reactions in biochemical pathways, but these reactions are inherently slow and the enzymes catalyzing them have evolved efficient strategies to lower the activation energy barrier. Cloudflare Ray ID: 5f7c3ed9ceaf2c76 Notice, however, the regiochemical outcome is different from the base-catalyzed reaction. The amine that reacts will Hinsberg's reagent to give an alkali insoluble product is : Assertion: $(CH_3)_2NH$ is less basic than $(CH_3)_3N$ in aqueous solution.
is reacted with HI ? When a nucleophilic substitution reaction involves a poor leaving group and a powerful nucleophile, it is very likely to proceed by an SN2 mechanism. These are both good examples of regioselective reactions. Both acid anhydrides and acid halides readily react with water and cannot exist for any length of time in living organisms. Both are highly reactive to acyl substitution reactions, and are often referred to as ‘activated acyl groups’ or ‘activated carboxylic acids’. The mechanism for the Sn\HCl reduction involves reductive electron transfer of the nitro group while the tin is being oxidized. ), Virtual Textbook of Organic Chemistry. Aniline 3. CBSE board exam 2021 preparation tips to ace the exam. These reactions can take place in either acidic or basic or neutral medium. explain the difference in reactivity towards nucleophiles of two or more given carboxylic acid derivatives. This leads to "two" epoxides. 2. Phenol. Like in other SN2 reactions, nucleophilic reactions take place with backside orientation relative to the leaving group, resulting in inversion at the electrophilic carbon. Carboxylic acid derivatives and acyl groups. Practice and master your preparation for a specific topic or chapter. The presence of these ions is used to balance the redox reaction equation. Which of the following set of molecules will have zero dipole moment ? The most reactive of the carboxylic acid derivatives frequently found in biomolecules are the acyl phosphates. As a general rule, the carbonyl carbon in an acyl group is less electrophilic than that in an aldehyde or ketone. As we will see in later sections of this chapter the hydrolysis of esters and amides are particularly important reaction types in biochemical pathways. There is a mirror plane, shown here. Delhi Govt. Identify product when (R) - and (S) -2 - butanol reacts with (R, R) tartaric acid in acidic medium. bhi. On electrolysis of dil.sulphuric acid using Platinum (Pt) electrode, the product obtained at anode will be: An element has a body centered cubic (bcc) structure with a cell edge of 288 pm. Common benzene reactions are Nitration of Benzene. Time it out for real assessment and get your results instantly. Ring-opening reactions can proceed by either SN2 or SN1 mechanisms, depending on the nature of the epoxide and on the reaction conditions. We will then discuss how thioesters play a key role in the acyl substitution reactions of lipid metabolism. 3. Electrolytic reduction of nitrobenzene in weakly acidic medium gives : Nitrobenzene on electrolytic reduction in strongly acidic medium gives, JEE Main 2021: January Session likely to be postponed to February. Chemistry . However, these two mirror images are actually identical due to the mirror plane of the cis geometry. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. This reaction is known as nitration of benzene. • Amides do undergo acyl substitution reactions in biochemical pathways, but these reactions are inherently slow and the enzymes catalyzing them have evolved efficient strategies to lower the activation energy barrier. Cloudflare Ray ID: 5f7c3ed9ceaf2c76 Notice, however, the regiochemical outcome is different from the base-catalyzed reaction. The amine that reacts will Hinsberg's reagent to give an alkali insoluble product is : Assertion: $(CH_3)_2NH$ is less basic than $(CH_3)_3N$ in aqueous solution.